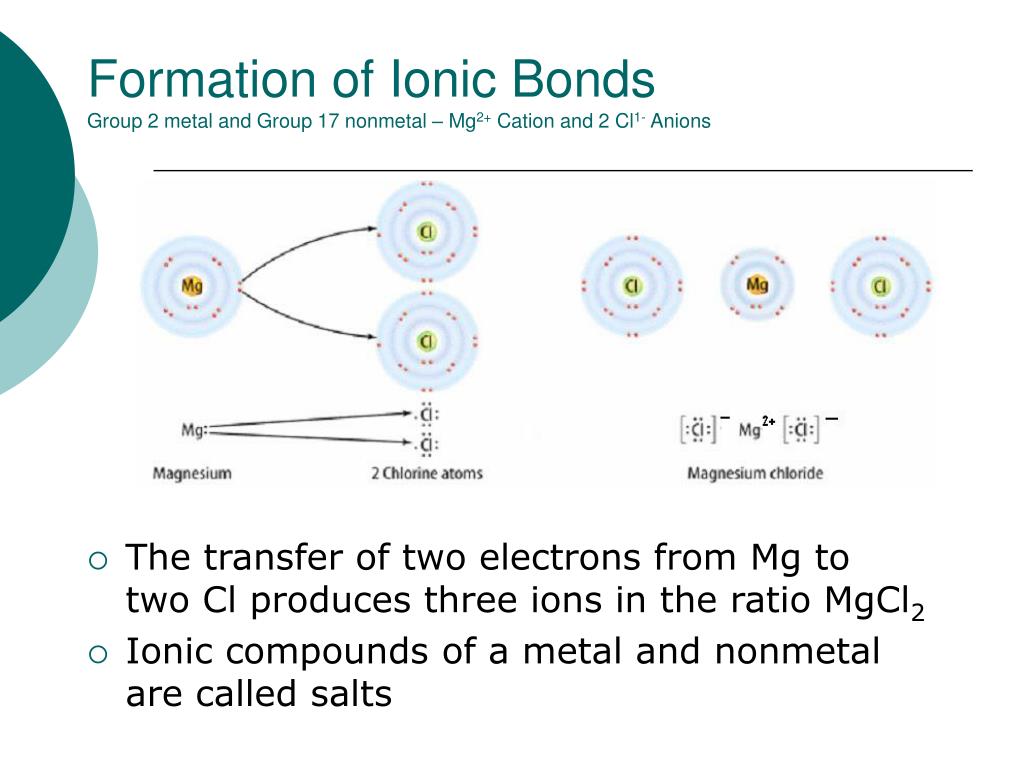
Are Ionic Bonds Metal And Nonmetal . A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. A metal (which forms the cations) and a nonmetal (which forms the. binary ionic compounds are composed of just two elements: a compound that contains ions and is held together by ionic bonds is called an ionic compound. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. The periodic table can help us. Ionic bonds are formed between cations and anions. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals.
from www.slideserve.com
By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. A metal (which forms the cations) and a nonmetal (which forms the. The periodic table can help us. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. binary ionic compounds are composed of just two elements: ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. a compound that contains ions and is held together by ionic bonds is called an ionic compound. Ionic bonds are formed between cations and anions.
PPT Chemistry IANB Page 11 PowerPoint Presentation, free download ID3818525
Are Ionic Bonds Metal And Nonmetal binary ionic compounds are composed of just two elements: ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. The periodic table can help us. binary ionic compounds are composed of just two elements: A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. Ionic bonds are formed between cations and anions. a compound that contains ions and is held together by ionic bonds is called an ionic compound. A metal (which forms the cations) and a nonmetal (which forms the.
From slideplayer.com
Ch. 7 Ionic Bonds. ppt download Are Ionic Bonds Metal And Nonmetal The periodic table can help us. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. A metal (which forms the cations) and a nonmetal (which forms the. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals.. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT Ionic Compounds Formula to Name PowerPoint Presentation, free download ID6776451 Are Ionic Bonds Metal And Nonmetal ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. The periodic table can help us. binary ionic compounds are composed of just two elements: By definition,. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT ATOMS PowerPoint Presentation, free download ID2618827 Are Ionic Bonds Metal And Nonmetal The periodic table can help us. Ionic bonds are formed between cations and anions. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. A metal (which forms the. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT Chemistry IANB Page 11 PowerPoint Presentation, free download ID3818525 Are Ionic Bonds Metal And Nonmetal Ionic bonds are formed between cations and anions. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. binary ionic compounds are composed of just two elements: . Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Bonding Ionic bond (formula units) Between metal and a nonmetal ppt download Are Ionic Bonds Metal And Nonmetal Ionic bonds are formed between cations and anions. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the. a compound that contains ions and is. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Chapter 5 Lecture presentation ppt download Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT Noble Gases and Valence e Ionization Energy and Bonding PowerPoint Presentation ID5875405 Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. A cation is formed when a metal ion loses a valence electron while an anion is formed when a. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Bonding Ionic bond (formula units) Between metal and a nonmetal ppt download Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. a compound that contains ions and is held together by ionic bonds is called an ionic compound. A. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Chemistry Unit 4 Bonding ppt download Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. binary ionic compounds are composed of just two elements: ionic bonding results in compounds known as ionic, or electrovalent,. Are Ionic Bonds Metal And Nonmetal.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. a compound that contains ions and is held together by ionic bonds is called an ionic compound. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. The. Are Ionic Bonds Metal And Nonmetal.
From studynympholept.z21.web.core.windows.net
A Covalent Bond Forms Between Two Metals Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. Ionic bonds are formed between cations and anions. The periodic table can help us. binary ionic compounds are composed of. Are Ionic Bonds Metal And Nonmetal.
From slidetodoc.com
BASIC CHEMISTRY Chapter 2 Advanced Human Anatomy Introduction Are Ionic Bonds Metal And Nonmetal A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. Ionic bonds are formed between cations and anions. A metal (which forms the cations) and a nonmetal (which forms the. a compound that contains ions and is held together by ionic bonds is called an ionic compound. By definition,. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT CHAPTER 2 A tomic structure and interatomic bonding PowerPoint Presentation ID5479764 Are Ionic Bonds Metal And Nonmetal A metal (which forms the cations) and a nonmetal (which forms the. binary ionic compounds are composed of just two elements: By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. Ionic bonds are formed between cations and anions. ionic bonding results in compounds known as ionic, or electrovalent,. Are Ionic Bonds Metal And Nonmetal.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Are Ionic Bonds Metal And Nonmetal binary ionic compounds are composed of just two elements: ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. a compound that contains ions and is held together by ionic bonds is called an ionic compound. A cation is formed when a metal ion loses a. Are Ionic Bonds Metal And Nonmetal.
From slidetodoc.com
Bonding Between Atoms Why Do Atoms Form Bonds Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. The periodic table can help us. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Chemical Bonding. ppt download Are Ionic Bonds Metal And Nonmetal A metal (which forms the cations) and a nonmetal (which forms the. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. a compound that contains ions and is held together by ionic bonds is called an ionic compound. binary ionic compounds are composed of just two elements: A. Are Ionic Bonds Metal And Nonmetal.
From www.youtube.com
Predicting bond type (metals vs. nonmetals) AP Chemistry Khan Academy YouTube Are Ionic Bonds Metal And Nonmetal A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. A metal (which forms the cations) and a nonmetal (which forms the. binary ionic compounds are composed of just two elements: By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT Ion Charge and the Formulas of Ionic Compounds PowerPoint Presentation ID2068191 Are Ionic Bonds Metal And Nonmetal ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. a compound that contains ions and is held together by ionic bonds is called an ionic compound. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non.. Are Ionic Bonds Metal And Nonmetal.